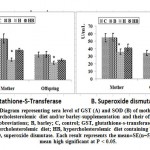
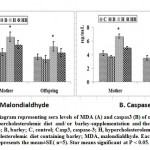
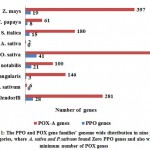
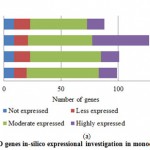
Introduction
Diabetes mellitus is a set of disorder that is mainly caused by impairment of insulin production.1 The second cause is a complication that involves poor insulin secretion due to the low action of the β-cell.2Based on the contemporary records about the shifting age structure of the global population of these omnipresent persistent debilitating menace among adults as 8.5% which doubled the 4.7% rate during 1980, albeit in retrospect, the world wide diabetes record during 1980 was documented as 108 million which reached 382 million during the year 2013 which flared and increased to 422 million during the year 2016.3 The vital cell’s failure to detect high blood sugar keeps insulin levels low.2DM derails metabolism in several organs such as the eyes and the kidneys.4 Insulin resistance in the human body also results in diabetes; this is a case where cells continue to absorb sugar because they have become insensitive to the pancreatic secreted hormone. When the liver absorbs sugar even when the levels are above normal, it causes liver and diabetes complications.5
One of the complications that result from insufficient insulin is lipid impairment, whereby lipoproteins develop insensitivity to insulin,6,7 A variety of complications emanating from variegated symptoms including high blood sugar, frequent urination, increased hunger which, if left untreated, diabetes can cause many deleterious damage.8 Moreover, serious long term implications embodying diabetic ketoacidosis (DKA), hyperosmolar hyperglycemic state, or unavoidable tragic death. Severe long term implications could result in devastating ailments embodying cardiovascular disease, stroke, kidney disease (CKD), foot ulcers, and damage to the eyes. Kidney and nervous system complications emanate from mainly high effects of blood sugar rather than hypoglycemia.9 The permanency of elevated glucose gradually erodes the functionality of the kidneys and induces poor responsiveness of the nerves. This complication has the capability to cause inefficiency of the organs and their complete dysfunction.9
For centuries, many parts of the world have documented the use of plants as direct sources of medicine. In South Asia, over 100 traditional communities each with its own set of herbs have relied on herbal extracts as multiple-disease remedies.10 Avicennia marina (Forssk Vierh) is an important mangrove species and one of the most widely distributed genera.11 The genus Avicennia named after Islamic physician Abdallah Ibn Sina,12 Owing to their breathing aerial roots (pneumatophores), the species like A. marina have been traditionally classified in the family of Verbenaceae,13 but their real family is Acanthaceae.14 Because of existence in very harsh conditions, in dry areas with high salinity, such as on the eastern and western coasts of the Red Sea, the leaves of A. marina have acquired adaptational features that change with each environment.13
Avicennia marina is the most widely distributed mangrove species across all genera in the world. It extends from the shoreline of Egypt, along East Africa, through the southern parts of the continent, along with the Red Sea and in the Oceania and Pacific regions including Australasian coasts and the Japanese shoreline.11 This wide geographical reach owes to the plant’s highly regarded adaptational abilities, such as affinity for heat levels above 35 °C and advanced saltiness, for example in the Arabian Peninsula.15 The objectives of the present investigation were to assess the antihypoglycemic ramifications of aqueous extracts of A. marian against experimentally STZ-induced kidney damage.
Materials and Methods
Experimental Animals
The experiment was performed on 60 adult male Wistar albino rats (200 to 250g average body weight). The animals were housed in well-aerated individual cages in an animal room and maintained at constant temperature (24 ± 1°C) and humidity (55 ± 10%) with alternating 12 h light/ dark cycle. The rats were fed with normal commercial chow and water ad libitum. The animals were maintained in accordance with the international ethical guidelines for the care of laboratory animals and all experimental procedures were approved by the Animal Care and Use Committee of the King Abdulaziz University.
Plants Extraction
The collected leaves of A. marina plants were scientifically identified and authenticated by a plant taxonomist at the Department of Arid Land Agriculture, Faculty of Meteorology, Environment and Arid Land Agriculture, King Abdulaziz University, Jeddah, Saudi Arabia. Aqueous extracts of plant leaves were prepared according to previous reporters.16,17
Preparation of STZ and Induction of Diabetes
Overnight-fasted adult male rats (6 weeks old) were intraperitoneally injected with a single dose (60 mg/kg) of freshly prepared STZ.18 Three days after STZ injection, the fasting blood glucose levels were measured in blood samples taken from rats’ tail by using a One Touch Ultra Glucometer (Lifescan, Johnson and Johnson, Milpitas, CA, USA). Animals with blood glucose levels ≥ 250 mg/dl were considered diabetic and used for the experiment19. This day was considered the first day of the experiment.
Experimental Design
The experimental rats were randomly divided into 4 equal groups, each of 15 rats. Group 1: Normal rats received water and fed ad libitum .Group 2: STZ-induced diabetic rats. Groups 3: diabetic rats treated orally with aqueous leaf extracts of A. marina (400 mg/kg BW/day), Group 4: Non-diabetic rats received an aqueous leaf extract of A. marina at a dose of 400 mg/kg BW/day. The treatments started on the 4th day after STZ injection, which considered as the 1st day of treatment, and continued daily for six weeks.
Measurement of Blood Glucose Levels
To measure the glucose levels, fresh fasting blood samples were collected from the rat’s tail vein, and glucose levels were then determined using a One Touch Ultra Glucometer (Lifescan, Johnson and Johnson, Milpitas, CA, USA).
Measurement of Serum Glucose and Insulin
Blood was collected from retro-orbital venous plexus of rats at the 6th week, left for clotting at room temperature and serum was separated by centrifugation at 3000 rpm for 20 min. Serum glucose and insulin levels were determined at the 6th week post- treatments by commercial kits (Roche cobas Diagnostics USA) using cobas 6000 analyzer series. Serum insulin levels were measured using insulin ELISA kits which includes an enzyme immunoassay for the quantitative determination of insulin in sera of rats (Cat. no. ezrmi-13kelisa, Billerica, MA, USA) according to a method.19
Measurement of Serum Creatinine, Urea and Uric Acid
Assessment of Serum Creatinine
The serum creatinine level was determined according to Henry et al.20
Principle of the Test
Creatinine reacts with picric acid in alkaline condition to form a yellow-orange complex which is measured at 492 nm. The rate of formation of color is proportional to creatinine quantity in the sample.
![Vol36No3_Imm_IMA_Img1]()
Calculation
![Vol36No3_Imm_IMA_Img2]()
Where
Absorbance of the sample.
Absorbance of the standard.
To convert the result (mg/dL) to umol/L divide by 88.4
Assessment of Serum Blood Urea Nitrogen (BUN).
The serum BUN level was determined according to Henry et al.20
Principle of the Test
Urea is synthesized in the liver from the ammonia produced mostly by the catabolism of amino acids. Kinetic enzymatic estimation of urea uses these reactions.
![Vol36No3_Imm_IMA_Img3]()
Urease hydrolyses urea to ammonia. Glutamate dehydrogenase (GLDH) combines the ammonia with 2-oxoglutarate to form glutamate. In this reaction, the NADH is oxidized to NAD+ and this change is detected photometrically as a decrease in absorbance at 340 nm (Warburg’s optical test).
Calculation
![Vol36No3_Imm_IMA_Img4]()
Where: n = 50.0 mg/dL (8.33 mmol/L).
Assessment of Serum Uric Acid
The method of Young21 was used to determine the level of uric acid.
Principle of the Test
Uric Acid is oxidized by uricase to produce allantoin and H2O2. 2-Hydroxy-2, 4, 6-tribromobenzoic acid (TBHBA) + 4-aminophenazone (4-AAP) + H2O2, in the presence of POD, produces a colored chromagen that is measured at 520 nm. The color intensity at 520 nm is proportional to the concentration of uric acid in the sample.
![Vol36No3_Imm_IMA_Img5]()
Calculation
![Vol36No3_Imm_IMA_Img6]()
Where:
A is absorbance of the sample or standard.
To convert the result (mg/dL) to mmol/L divide
Histopathological Examinations
The kidney tissue samples were collected at the 6th week post-treatments after sacrifice and fixed in 10% neutral formalin. After fixation, the samples were embedded in paraffin, 5-μm sections were cut, stained with hematoxylin and eosin (H&E), and then examined for pathological studies using light microscopy.22
Immunohistochemical Study
The standard immunohistochemical methods were adopted for detection of apoptotic caspase3 and diabetic insulin biomarkers in pancreatic tissue.23 The tissue sections were routinely microwave-treated to unmistaken the epitopes of antigen.24 The Biotin-Streptavidin (BSA) system was used to visualize the apoptotic and insulin markers.25 Diaminobenzidine (DAB) was used as chromogen since it allows a permanent preparation. Hematoxylin counterstain was done.
Statistical Analysis
The obtained data in this study were expressed as mean ± standard error (SE). Statistical significance of the difference between groups, with more than two categories, was determined by one-way analysis of variance (ANOVA) followed by Least Significant Difference (LSD) post-hoc test. The statistical software package used for analysis was Statistical Package for So
The kidney tissue samples were collected at the 6th week post-treatments after sacrifice and fixed in 10% neutral formalin. After fixation, the samples were embedded in paraffin, 5-μm sections were cut, stained with hematoxylin and eosin (H&E), and then examined for pathological studies using light microscopy.22
Immunohistochemical Study
The standard immunohistochemical methods were adopted for detection of apoptotic caspase3 and diabetic insulin biomarkers in pancreatic tissue.23 The tissue sections were routinely microwave-treated to unmistaken the epitopes of antigen.24 The Biotin-Streptavidin (BSA) system was used to visualize the apoptotic and insulin markers.25 Diaminobenzidine (DAB) was used as chromogen since it allows a permanent preparation. Hematoxylin counterstain was done.
Statistical Analysis
The obtained data in this study were expressed as mean ± standard error (SE). Statistical significance of the difference between groups, with more than two categories, was determined by one-way analysis of variance (ANOVA) followed by Least Significant Difference (LSD) post-hoc test. The statistical software package used for analysis was Statistical Package for Social Sciences (SPSS 24). The values were considered to be significantly different when the P value was < 0.05.26
Results
Fasting Blood Glucose
Our final results confirmed that while fasting, the level of blood glucose (mg/dL) of STZ-induced diabetic animals (G2) and STZ-induced diabetic rats administered A.marina extract (G3) remained significantly elevated (p ≤ 0.001) compared to normal control group (G1). Daily oral treatment of STZ-induced diabetic rats (G3) with A.marina leaf extract induced non-significant changes in blood glucose levels as compare to diabetic control group (G2) (Figure 1)
Results
Fasting Blood Glucose
Our final results confirmed that while fasting, the level of blood glucose (mg/dL) of STZ-induced diabetic animals (G2) and STZ-induced diabetic rats administered A.marina extract (G3) remained significantly elevated (p ≤ 0.001) compared to normal control group (G1). Daily oral treatment of STZ-induced diabetic rats (G3) with A.marina leaf extract induced non-significant changes in blood glucose levels as compare to diabetic control group (G2) (Figure 1)
Serum Glucose
A comparison of serum glucose differences are illustrated in Figure 2 The serum glucose level (mmol/L) of STZ-induced diabetic rats (G2) and STZ-induced diabetic rats treated with A.marina extract (G3) showed highly significant increase (p ≤ 0.001) as compared to the normal control group (G1). However, the treatment of diabetic rats (G3) with A.marina leaf extracts revealed non-significant changes in the serum glucose levels in comparison to STZ-induced diabetic rats (G2).
Serum Insulin
Figure 3 exhibited a highly significant decrease (p ≤ 0.001) in serum insulin level (mlU/L) of STZ-induced diabetic rats (G2) and treated diabetic animals (G3) compared to the normal control group (G1). The treatment of diabetic groups (G3) with A.marina exhibited non- significant changes in the serum insulin levels compared to STZ-induced diabetic rats (G2).
Serum Creatinine
The levels of serum creatinine (μmol/L) in STZ-induced diabetic rats (G2) revealed a significant increase (P ≤ 0.01) compared to the normal control group (G1). However, the treatment of diabetic rats (G3) with A.marina leaf extracts revealed non-significant change in the serum creatinine levels in comparison to normal control group (G1). Nevertheless, the treatment of the STZ-induced diabetic rats (G3) with A.marina exhibited non-significant change in the serum creatinine levels when comparison was made to the diabetic control group (G2) (Figure 4).
Serum Blood Urea Nitrogen (BUN)
The serum BUN levels (mmol/L) of the STZ-induced diabetic rats (G2) showed a highly significant increase (P ≤ 0.001) compared to the normal control rats (G1). Similarly, the STZ-induced diabetic rats treated with A. marina (G3) demonstrated a highly significant increase (P ≤ 0.001) in the levels of serum BUN when compared with the normal control rats (G1). However, administration of A. marina to diabetic rats (G3) showed a non-significant difference in the serum BUN level compared to diabetic control group (G2) (Figure 5).
Serum Uric Acid
A comparison of serum uric acid levels (umol/L) was illustrated in Figure 6. Data recovered from the serum uric acid levels of STZ-induced diabetic rats (G2) showed a significant increase (P ≤ 0.01) as compared with the normal control group (G1). However, the treatment of diabetic rats (G3) with A.marina leaf extracts revealed non-significant change in the serum uric acid levels in comparison to normal control group (G1). On the other hand, daily oral administration of A.marina leaf extracts to STZ-induced diabetic rats (G3) resulted in a significant decrease (P ≤ 0.05) in serum uric acid levels as compared with the diabetic control group (G2).
The daily oral administration of A. marina to non-diabetic rats (G4) induced non-significant changes in the levels of blood glucose, serum glucose, creatinine, blood nitrogen urea and uric acid compared to normal control group (G1) (Figure 1-6).
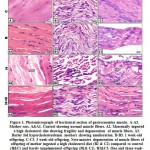
Histopathological Examinations of Kidney Tissue
Renal parenchyma and stroma of normal rats (G1) were normal with keeping features of the nephron units, collecting tubules, papillary and pelvic structures (Figure 7). But renal serial sections of STZ-induced in diabetic rats (60 mg STZ/kg BW, Single ip) (G2) revealed that moderate an array of multifocal necrotic areas (coagulative necrosis) with pyknotic or karryoretic nuclei and deep eosinophilic cytoplasm with keeping a ghost of the tubular and glomerular architecture and distinct inflammatory reaction (Figure 8). Additionally, variable degrees of degenerative changes including cloudy swelling, vacuolar and hydropic degeneration were noticed. Besides this, mild dilatation of some distal convoluted tubules and collecting tubules with partial atrophy of their lining epithelium was recorded. Moreover, a few apoptotic cells were seen in the tubular epithelium together with peculiar perivascular edema and mild to moderate congestion of intertubular and glomerular blood vessels and capillaries, sometimes with erythrocytic extravasations (Figure 8).
Renal lesions of STZ-induced diabetic rats (60 mg STZ/kg BW, single ip) treated with A. marina 400 mg/kg BW (daily oral dose for 6weeks) (G3) were represented by characteristic perivascular edema with vacuolation of the vascular walls. Some of the nephron units (glomeruli and tubules) were apparently normal, but others showed degenerative and early necrotic and apoptotic changes. The renal papillae and pelvis were apparently normal in most parts; however, a few of them showed mild vascular and tubular dilatations of the surrounding tissues, besides focal exfoliative change in the lining epithelium (Figure 9)
Sections from kidneys of non-diabetic rats, treated with A. marina, 400 mg/kg BW (daily oral dose for 6weeks) (G4), revealed apparently normal nephron units including glomeruli and different types of tubules. The renal papillae, pelvis, medullary rays and blood vessels were also apparently normal. However, a few renal tubular epithelia, especially in the cortex, showed mild degenerative changes, mainly hydropic degeneration (Figure 10).
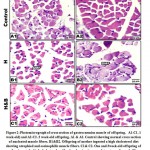
Immunohistochemical Kidney Findings
Kidney sections of normal rats (G1) denoted normal parenchyma free from any apoptotic changes except for a very few cells in the medullary collecting tubules (0.5-1%) /HPF (Figure 11). Meanwhile, renal tissue of STZ-induced diabetic (60 mg STZ/kg BW, Single ip dose) (G2) revealed about 3-5% positive apoptotic reaction of the tubular epithelium, particularly in the medulla (Figure 12). However, kidney sections of STZ-induced diabetic rats (60 mg STZ/kg BW, single ip dose) treated with A. marina 400 mg/kg BW (daily oral dose for 6 weeks) (G3) revealed about 6-8% /HPF of the tubular epithelial cells and 3-5% /HPF of the glomerular cells with positive cytoplasmic apoptotic reaction (Figure 13). Concomitantly, Some of the renal sections of non-diabetic rats treated with A. marina, 400 mg/kg BW (daily oral dose for 6 weeks) (G4) were free of apoptosis, but other sections showed 4-6% cells/HPF of both tubular and glomerular cells with early apoptotic changes as proved by weak brownish cytoplasmic reactivity to caspase-3 (Figure 14).
Discussion
Diabetes mellitus is a common metabolic disorder, characterized by hypoinsulinemia, hyperglycaemia, hyperlipidemia, hyperaminoacidemia, and disturbances of acute impact on carbohydrates, proteins and lipid metabolisms resulting from serious deleterious defects in either insulin secretion, insulin action, or both.27,28 Remedies developed on the principles of chemical and conventional medications are usually of short affectivity, with a risk of adverse side effects and are usually of high prices, particularly for third world populations. Consequently, treatment of DM with plant derived phytochemicals that are easily obtained and are relatively cheap appears extremely likable.29 Several investigations were applied to review the effectiveness of the plant extracts on diabetes evoked by STZ in many experimental animal tissues.30,31 Many natural products can suppress the enzyme activities responsible for the production of glucose, its absorption, and insulin effectiveness.32,33Other studies have clarified those positively effective components in plant extracts that can modify apoptosis of β-cell and promote insulin action.34-36 The anti-diabetic effects of A. marina have been explored in many studies.37,38
In our current investigation, diabetes was induced by the administration of a single dose of STZ (60 mg/kg B.W), tested animals with blood glucose levels above 250 mg/dl on the 3rd day post treatment of the STZ injection were regarded as diabetic rats.19 In this study, the dramatic elevation of the fasting blood glucose and serum glucose levels (at 6th week) after induction of diabetes were encountered in the diabetic rats as compared with the normal control rats. The treatment of STZ-induced diabetic rats with A. marina revealed non-significant changes in blood and serum glucose levels in comparison with the diabetic control group. Streptozotocin is a cytotoxic compound especially toxic to the pancreatic β-cells, which are responsible for the production of insulin in mammals. It enters pancreatic β-cells through GLUT2 channels in the cellular plasma membrane, which evidently leads to cellular toxicity and local immune reactions leading finally to hypoinsulinemia and hyperglycemia in animals.39 The harmful effect of STZ on β-cells leads to the development of deficient production of insulin and thus, the elevation of blood glucose level occurs23. Moreover, a significant increase of serum/plasma glucose could provoke additional destruction of β-cells of the pancreas.40
Insulin is the playmaker in diabetes of all types and has a crucial function in glucose homeostasis. All patients with type-1 diabetes would like to have hormone treatment for good health (insulin-dependent) unless they receive islet cells or whole organ transplant; several patients with type-2 diabetes could need this hormone once their β-cell function declines over time41. The findings of this study indicated that, at the end of the experimental period, the serum insulin levels were significantly decreased in STZ-induced diabetic rats as compared with the normal control ones. Administration of aqueous extracts of A. marina to diabetic rats resulted in a non- significant change in serum insulin levels when compared with the diabetic control group.
Moreover, creatinine is a well-authenticated as a metabolic derivative of muscle creatine and phosphocreatine and its concentration in serum is proportional to the body muscle mass. Therefore, the amount of creatinine is usually stable and its elevated levels indicate diminished renal function, as it is easily excreted by the nephrons.42 Low serum creatinine levels are positively associated with an increased risk of incident dysglycemia.43,44 Likewise, other studies recorded a positive association between serum creatinine and diabetes prevalence.45 In the current study, there was a significant increase in serum creatinine level of STZ-induced diabetic rats as compared with the normal control rats. The daily administration of aqueous extract of the investigated plants has a no impact on serum creatinine of diabetic animals as compared with STZ-induced diabetic rats. In contrast, Gandomani and Malati46 indicated that A. marina at a dose of 400 mg/kg significantly decreases serum pro-inflammatory cytokines in rats with a consequent reno-protective effect.
It was formally documented that urea is the end product of protein catabolism in the living system and hence, it is synthesized in the liver from ammonia which is produced consequently as a result of the deamination of amino acids. Diabetic nephropathy is one of the major causes of chronic renal failure which is associated with an increased level of BUN and creatinine.45 Another urgent factor is uric acid which is the metabolic end product of purine catabolism and its elevation levels in serum signifies renal functional impairment. Serum uric acid level has been suggested to be associated with the risk of type-2 diabetes. Albeit, biologically, uric acid plays an important role in weakening of insulin resistance in animal models by suppressing the bioavailability of nitric oxide, which is essential for insulin-stimulated glucose uptake.47
In the current study, our evaluation documented a significant increase in a cascade manner embodying increase in serum blood urea nitrogen and uric acid levels of STZ-induced diabetic rats as compared with normal rats. Treatment of STZ-induced diabetic rats with A. marina revealed non-significant change in serum blood urea nitrogen in comparison to the diabetic control group. The results of the current study are contrary to that obtained by Mirazi et al.48 The authors concluded that hydro-alcoholic extract of A. marina leaves at doses of 400mg or 800mg/kg. B.W could be able to treat renal toxicity induced by CCl4 in male rats with a significant reduction of urea. On the other hand, daily oral administration of A. marina extract to STZ-induced diabetic rats resulted in a significant decrease in serum uric acid levels in comparison to the diabetic control group
Diabetic nephropathy is a major complication of DM and a leading instigator of end-stage renal failure, as a worldwide calamity. The severity of diabetic nephropathy is one of the major factors determining the prognosis of diabetic patients.49 The current investigation on the histo-morphology of the kidney of normal rats showed normal structures of nephron units in almost all the examined sections with keeping features of normal glomeruli, proximal and distal convoluted tubules, loops of Henle, collecting tubules, renal papillae, and renal pelvis. Moreover, the renal blood vessels and intertubular capillaries were in good condition. Renal sections of STZ-induced diabetic rats revealed multifocal coagulative necrotic areas with a pyknotic or karyopyknotic nuclei, deep eosinophilic cytoplasm, and distinct inflammatory reaction. Variable degrees of degenerative changes with mild dilatation of some distal convoluted and collecting tubules along with the partial atrophy of their lining epitheliums were also recorded. Peculiar perivascular edema and mild to moderate congestion of intertubular and glomerular blood vessels and capillaries with erythrocytic extravasations were also seen.
The results of the current study are partially similar to that obtained by Zafar et al.50 who found large aggregates of lymphocytes in the interstitium of rat’s renal tissue following single IP injection of STZ (45 mg/kg B.W). The authors added that an inconstant number of proximal convoluted tubules showed signs of renal-tubular necrosis; their epithelial lining was disrupted and discontinued with pyknotic nuclei, vacuolated cytoplasm, broken cellular membranes and disappearance of brush borders. Nephropathic lesions of the current investigation coincide with that of Balamash et al.51 This battery of astute scientists have observed that the renal tissue of normal rats showed normal cortex and medulla were intact; renal corpuscles being the main feature of the cortex. Moreover, the outer layer of Bowman’s capsule was lined by simple squamous epithelium and the glomerulus looked like cluster of capillaries covered by the inner layer of Bowman’s capsule. Furthermore, renal tubules in the cortex mainly include the proximal and distal convoluted tubules and these tubules were lined by the cuboidal epithelium.
Kidney lesions in STZ-induced diabetic rats treated with A. marina were represented by characteristic perivascular edema with vacuolation of the vascular walls. Some of the nephron units (glomeruli and tubules) were normal; meanwhile, others showed degenerative and early necrotic changes. The renal papillae and pelvis were normal in most parts; however, a few of them showed mild vascular and tubular dilatations of the surrounding tissue, besides, focal exfoliative changes in the lining epithelium were also seen. With such lesions, a speculated mild decrease in the renal filtration threshold along with a consequent insignificant increase in the creatinine, urea, and uric acids could be expected.
The characteristic renal lesions in the current investigation could lead to induction of dramatic changes in the renal biochemical markers. Such changes could be attributed to the direct nephrotoxic effect of STZ compound and the hyperglycemic secondary toxic metabolites. This information is in agreement with what was revealed earlier that diabetic nephropathy is a major complication of DM and a leading cause of end-stage renal failure worldwide.52 A fact that led to the severity of diabetic nephropathy is one of the major factors determining the prognosis of diabetic patients with the subsequent results that consider it a major determinant of morbidity and mortality in patients with DM. Therefore, previous scientific reports showed that diabetic nephropathy was induced by hyperglycemia via several mechanisms such as oxidative stress along with induction and increase of glycation reaction. Henceforth, the production of highly reactive oxygen radicals was attributed to the hyperglycemic oxidative stress which could lead to prominent cellular cyto-toxicity in different tissues including kidney.53 Moreover, various investigations showed that the level of lipid peroxides as MDA was elevated both in serum and kidney tissues and the levels of the anti-oxidant enzyme were decreased in renal homogenates of diabetic rats.54
Diabetic renal pathologic lesions are contributed to the angio-bio-physiologic complication of DM, which leads to chronic renal problems.55 Apoptosis probably contributes to provoke diabetic nephropathy.56 Apoptotic protein p53 and active caspase-3 are major biomarkers of apoptosis.57 Oxidative stress can activate p53 leading to its nuclear accumulation resulting in cell cycle arrest, apoptosis, or cell removal from the proliferative pool.57 Apoptotic p53 up-regulation is reported in several different tissues in DM, including kidney.59,60 Apoptotic changes were induced by p53 through stimulation of caspases.61 Caspases constitute a group of cysteine proteases which exist in the cell as an inactive zymogen.62 Caspase-3 is the executioner caspase of apoptosis that becomes activated upon cleavage in the apoptotic process leading to the morphological features of apoptosis.63 Elevated levels of active caspase-3 are reported in many renal diseases.64,65
Kidney sections from different experimental groups of the present study revealed 6-8%/HPF in the tubular epithelial cells together with 3-5%/HPF in the glomerular cells of STZ-induced diabetic rats treated with A. marina. The observations of the current study are generally similar to that obtained by Al-Rasheed et al.66 The authors reported that apoptotic markers (BAX and caspase-3) were significantly increased and the anti-apoptotic marker (BCL-2) was decreased in kidneys of STZ-induced diabetic rats. The authors added that topographical localization and double immunohistochemical analysis suggested that podocytes were the main apoptotic cells under these conditions, although endothelial and mesangial cells were also affected. This confirms and extends the previous observations of Susztak et al.56 The authors identified topographically cell type(s) undergoing apoptosis and it was consistent with findings of Meyer et al.67 and Steffes et al.68 where the authors reported that significant podocyte apoptosis could be observed in diabetic patients, parallel with the progression of renal disease. The findings of the present study are in partial agreement with the results obtained by Ahmed et al.69 The authors declared that the pro-inflammatory cytokine TNF-α and the apoptotic mediators (p53 and caspase-3) were remarkably decreased in the kidney of STZ-induced diabetic rats as a result of treatment with VDR activator (paricalcitol), while the expression of anti-apoptotic protein BCL-2 was increased. Furthermore, it should be mentioned consequently that in the aforementioned study, vitamin D was used as a synthetic chemical compound, but it can be naturally obtained from many phytochemical plant sources.70
Finally, the apoptotic p53 protein together with pro-apoptotic proteins were consequently transported into the mitochondria as they encourage an increase in the mitochondrial membrane permeability and lead to energize of cytochrome c, which adheres to the apoptotic protease activating factor 1 (APAF-1) and with the caspase-9 proenzyme, to form a complex called the “apoptosome”. The latter, consecutively, synergized caspase-9 with consequent stimulation of caspase-3 proenzyme to the protease stage, which then sticks to the effector caspase group. Effector caspase-3, -6, and -7 do not need an adaptor protein for dimerization-induced activation. Rather, effector caspases spontaneously dimerize but are only activated upon cleavage between the small and large subunits by an active initiator caspase. Activated effector caspases then cleave a number of protein substrates to initiate apoptosis leading to the subsequent dismantling of cellular components,71-74 evidently the effector caspases induce intracellular protein lysis and the morphological distinctive changes of apoptosis.75 It was reported that apoptosis could aggravate the pathomechanisms of diabetic nephropathy and nephrotoxicity through caspase-3 expression.76 The mitochondrial oncogene product BCL-2, prevented caspase-3 activation during a variety of proapoptotic conditions.77
Conclusion
The findings of this study evidently showed that the aqueous leaf extract of A. marina exerted protective effect against streptozotocin-induced nephrotoxicity in male rats as demonstrated by amelioration of the aforementioned biochemical parameters, histopathological and immunohistochemical changes in the kidney sections and restored renal tissue architecture near normal levels. The plant extract showed antihyperglycemic, insulinotropic, and renoprotective actions. Moreover, the findings of this investigation may lead to the development of novel anti-diabetic drugs through the use of up to date technologies which is described as a highly needed imperative
Funding source
There is no funding source
Conflict of Interest
The author of this study did not have any type of conflict of interest.
References
- American Diabetes Association, Standards of medical care in diabetes. Clin. Appl. Res. Edu. 2017; 40(1): 1049-5992.
- Cerf ME. β-cell dysfunction and insulin resistance. Endocrinol. 2013:4(37): 1-13.
- World Health Organization (WHO) and International Diabetes Federation (IDF), 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. WHO, Geneva.
- Riaz S. Diabetes mellitus. Res. Essays. 2009; 4(5): 367-373.
- Macdonald I. a review of recent evidence relating to sugars, insulin resistance, and diabetes. Eur. J. Nutr. 2016; 55(2): 17-23.
- Solano M.P., Goldberg R.B. Management of dyslipidemia in diabetes. Rev. 2006; 14(3): 125-135.
- Ndisang J.F., Vannacci A., Rastogi S. Insulin resistance, type 1 and type 2 diabetes, and related complications. Diabetes Res. 2017: 1-3.
- Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009; 32(7): 1335–1343.
- Chawla A., Chawla R., Jaggi S. Microvascular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian Endocrinol. Metab. 2016; 20(4): 546-551.
- Rahmatullah M., Mollik A.H., Azam A.T.M.A., Islam R., Chowdhury A.M., Jahan R., Chowdhury M.H., Rahman T. Ethnobotanical survey of the Santal tribe residing in thakurgaon district, Bangladesh. Eurasian J. Sustain. Agric. 2009; 3(4): 889-898.
- Said W.M., Ehsan N.O.M. Morphological and molecular evidence among four heteroforms of Avicennia marina (Forssk) vierh. Am. Sci. 2010; 6(11): 843-856.
- Quattrocchi U. CRC World Dictionary of Plant Names: common names, scientific names, eponyms, synonyms, and etymology. 4 vols. CRC Press, Boca Raton, FL. 2000; pp.2896.
- Sultana, R.S. Stem and leaf anatomy of Lantana camara-a plant of the Verbenaceae family. Int. J. Curr. Res. Biosci. Plant Biol. 2016; 3(1): 27-31.
- Thamizharasan S., Saravanan N.A. Antibacterial potential of mangrove plant Avicennia marina against a clinical pathogen. J. Zool. Stud. 2016; 1(7): 14-16.
- Almahasheer H., Duarte C.M., Irigoien X. Phenology and growth dynamics of marina in the central Red Sea. Sci. Rep. 2016; 6: 1-9.
- Sahoo, Mulla N.S., Ansari ZA., Mohandass C. Antibacterial activity of mangrove leaf extracts against human pathogens. Indian J. Pharm. Sci. 2012; 74: 348–351.
- Mohamadi J., Havasian M.R. The study of inhibitory effect of aqueous extract leaf of Avicennia marina (Hara) on Candida albicans, In Vitro. J. Pharm. Life Sci. 2017; 8: 5547-5551.
- Al-Hariri MT. Comparison the rate of diabetes mellitus induction using streptozotocin dissolved in different solvents in male rats. Comp. Clin. Path. Res. 2012; 1: 96 -99
- Gurudeeban S., Kaliamurthi S., Thirugnanasambandam R. Positive regulation of Rhizophora mucronata poir extracts on blood glucose and lipid profile in diabetic rats. Med. 2016; 2: 1-10.
- Henry R.J., Cannon D.C., Winkelman J.W. Clinical chemistry: principles and techniques, 11th Ed, Happer and Row Publishers, New York, 1974; pp. 1629.
- Young, D.S., 1997. Effects of preanalytical variables on clinical laboratory tests, 2nd Ed. Washington. DC: AACC Press.
- Fernández T., Suarez G., Pérez C.L, Acosta T., Clapes S. Influence of diabetes and gestation in blood biochemistry variables in Wistar rats. J. Anim. Sci. 2018; 2: 1-4.
- Pournaghi P., Sadrkhanlou R.A., Hasanzadeh S., Foroughi A. An investigation on body weights, blood glucose levels and pituitary gonadal axis hormones in diabetic and metformin treated diabetic female rats. Res. Forum. 2012; 3: 79-84.
- Mazhar F.M., Moawad K.M., Abdel-Gawad M.H. Evidence for a reversing effect of vitamin E or curcumin on some biochemical alterations associated with diabetes in pregnant rats and their fetuses. J. Zool. 2005; 44: 367-388.
- Song W., Levin D.S., Varkey J., Post S., Bermudez V.P., Hurwitz J., Tomkinson A.E. A conserved physical and functional interaction between the cell cycle checkpoint clamp loader and DNA ligase I of eukaryotes. Biol. Chem. 2007; 282: 22721-22730
- Zar J.H. Biostatistical analysis prentice-hall, eryelwood cliffs. N.J. 1996; pp. 663.
- Wadkar K.A., Magdum C.S., Patil S.S., Naikwade NS. Anti-diabetic potential and Indian medicinal plants. Herb. Med. Toxicol. 2008; 2: 45-50.
- Al-Attar A.M., Alsalmi FA. Effect of Olea europaealeaves extract on streptozotocin-induced diabetes in male albino rats. Saudi J. Biol. Sci. 2019; 26:118-128.
- Bordoloi R., Dutta K.N. A review on herbs used in the treatment of diabetes mellitus. Pharm. Chem. Biol. Sci. 2014; 2: 86-92.
- Torrico F., Cepeda M., Guerrero G., Melendez F., Blanco Z., Canelón D.J., Diaz B., Compagnone R.S., Suárez A. Hypoglycaemic effect of Croton cuneatus in streptozotocin-induced diabetic rats. Bras. Farmacogn. 2007; 17: 166-169.
- Almalki D,A,, Alghamdi SA. Hepatorenal protective effects of some plant extracts on experimental diabetes in male rats. J. Pharmacol. 2019; 15: 238-247.
- Schmidt B., Ribnicky D.M., Poulev A., Logendra S., Cefalu W.T., Raskin I. A natural history of botanical therapeutics. Metabolism 2008; 57: 3-9.
- Nazaruk J., Borzym-Kluczyk M. The role of triterpenes in the management of diabetes mellitus and its complications. Rev. 2015; 14: 675-690.
- Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T.P. Indian herbs and herbal drugs used for the treatment of diabetes. Clin. Biochem. Nutr. 2007; 40: 163-173.
- Oh Y.S., Jun H.S. Role of bioactive food components in diabetes prevention: effects on beta-cell function and preservation. Metab. Insights. 2014; 6: 51-59.
- Saisho Y. β-cell dysfunction: its critical role in prevention andmanagement of type 2 diabetes. World J. Diabetes. 2015; 6: 109-124.
- Revathi P., Jeyaseelan S.T, Thirumalaikolundusubramanian P., Prabhu N. An overview of antidiabetic profile of mangrove plants. J. Pharm. Pharm. Sci. 2014; 6: 1-5.
- Das S.K., Samantaraya D., Patrab J.K., Samantac L., Thatoid H. Antidiabetic potential of mangrove plants: a review. Front. Life Sci. 2015; 9: 75-88.
- Al-Nahdi A.M.T., John A., Raza H. Cytoprotective effects of N-acetylcysteine on streptozotocin-induced oxidative stress and apoptosis in RIN-5F pancreatic β-cells. Cell Physiol. Biochem. 2018; 51: 201-216.
- El-Desouki N.I., Tabl G.A., Abdel-Aziz K.K., Salim E.I., Nazeeh N. Improvement in beta-islets of Langerhansin alloxan-induced diabetic rats by erythropoietin and spirulina. Basic. Appl. Zool. 2015; 71: 20-31.
- McCulloch D.K, MD., Nathan D.M., MD., Mulder JE., M.D. General Principles of Insulin therapy in Diabetes Mellitus. UpToDate-Online Database. Updated March, 2016. Available at: http://www.uptodate.com/contents/general-principles-of-insulintherapy-in-diabetes-mellitus Accessed December 2017.
- Loeb S. Clinical laboratory test: values and implications. Copy right, Springhouse Corporation, Springhouse, Pennsylvania, 1991
- Nayak B.S., Butcher D.M., Bujhawan S., Chang D., Chang S., Cabral-Samaroo, Cadan S., Buchoon V., Budhram L., Boyce M., Teelucksingh S. Association of low serum creatinine, abnormal lipid profile, gender, age and ethnicity with type 2 diabetes mellitus in Trinidad and Tobago. Diabetes Res. Clin. Pract. 2011; 91: 342-347.
- Takeuchi M., Imano H., Muraki I., Shimizu Y., Hayama-Terada M., Kitamura A., Okada T., Kiyama M., Iso H. Serum creatinine levels and risk of incident type 2 diabetes mellitus or dysglycemia in middle-aged Japanese men: a retrospective cohort study. BMJ Open Diabetes Res. Care. 2018; 6: 1-7.
- Chutani A., Pande S. Correlation of serum creatinine and urea with glycemic index and duration of diabetes in type 1 and type 2 diabetes mellitus: a comparative study. J. Physiol. Pharm. Pharmacol. 2017; 7: 914-919.
- Gandomani MZ, Malati EF. Evaluation of protective efficacy of Avicennia marina (Forssk.) vierh leaves against complete freund᾽s adjuvant-induced arthritis in wistar. J. Pharm. Res. 2014; 13: 945-951.
- Khosla U.M., Zharikov S., Finch J.L., Nakagawa T., Roncal C., Mu W., Krotova K., Block E.R., Prabhakar S., Johnson R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67: 1739-1742.
- Mirazi N., Movassagh S.N., Rafieian-Kopaei M. The protective effect of hydro-alcoholic extract of mangrove (Avicennia marina) leaves on kidney injury induced by carbon tetrachloride in male rats. J. Nephropathol. 2016; 5: 118-122.
- Barton M., Sorokin A. Endothelin and the glomerulus in chronic kidney disease. Nephrol. 2015; 35:156-167.
- Zafar M., Naqvi S.N., Ahmed M., Kaimkhani, Z.A. Altered liver morphology and enzymes in streptozotocin-induced diabetic rats. J. Morphol. 2009; 27: 719-725.
- Balamash K.S., Alkreathy H.M., Al Gahdali E.H., Khoja S.O., Ahmad, A. Comparative biochemical and histopathological studies on the efficacy of metformin and Virgin olive oil against streptozotocin-induced diabetes in Sprague-Dawley rats. J. Diabetes Res. 2018; 2018: 1-10.
- Azarkish F., Hashemi K., Talebi A., Kamalinejad M., Soltani N., Pouladian N. Effect of the administration of Solanum nigrum fruit on prevention of diabetic nephropathy in streptozotocin-induced diabetic rats. Res. 2017;9: 325-332.
- Barrero A., Quilez-del-Moral J.F., Herrador M.M., Akssira M., Bennamara A., Akkad S., Aitigri M. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera Africana. Phytochemistry. 2004; 65: 2507-2515.
- Rouse, R.L., Stewart, S.R., Thompson, K.L., Zhang, J. Kidney injury biomarkers in hypertensive, diabetic and nephropathy rat models treated with contrast media. Pathol. 2013; 41: 662-680.
- Rheinberger M., Böger C.A. Diabetic nephropathy: new insights into diagnosis, prevention and treatment. Med. Wochenschr. 2014; 139: 704-706.
- Susztak K., Raff A.C., Schiffer M., Bottinger E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006; 55: 225-233.
- Erekat N.S. Cerebellar Purkinje cells die by apoptosis in the shaker mutant rat. Brain Res. 2017; 1657: 323-332.
- Horn H.F., Vousden K.H. Coping with stress: multiple ways to activate p53. Oncogene 2007:26:1306-1316.
- Qiu L.Q., Sinniah R., Hsu S.I. Coupled induction of iNOS and p53 upregulation in renal resident cells may be linked with apoptotic activity in the pathogenesis of progressive IgA nephropathy. Am. Soc. Nephrol. 2004:15: 2066-2078.
- Nakamura H., Matoba S., Iwai-Kanai E., Kimata M., Hoshino A., Nakaoka M, Katamura M., Okawa Y., Ariyoshi M., Mita Y., Ikeda K., Okigaki M., Adachi S., Tanaka H., Takamatsu T., Matsubara H. P53 promotes cardiac dysfunction in diabetic mellitus caused by excessive mitochondrial respiration-mediated reactive oxygen species generation and lipid accumulation. Heart Fail. 2012; 5: 106-115.
- Deshpande S.D., Putta S., Wang M., Lai J.Y., Bitzer M., Nelson R.G., Lanting L.L., Kato M., Natarajan R. Transforming growth factor-β-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes 2013; 62: 3151-3162.
- Fan T.J., Han L.H., Cong R.S., Liang J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. 2005; 37: 719-727.
- Erekat N.S. Active caspase-3 upregulation is augmented in at-risk cerebellar Purkinje cells following inferior olive chemoablation in the shaker mutant rat: an immunofluorescence study. Res. 2019; 41: 234-241.
- Bamri-Ezzine S., Ao Z.J., Londoño I., Gingras D., Bendayan M. Apoptosis of tubular epithelial cells in glycogen nephrosis during diabetes. Invest. 2003:83: 1069-1080.
- Yano T., Itoh Y., Matsuo M., Kawashiri T., Egashira N., Oishi R. Involvement of both tumor necrosis factor-alpha-induced necrosis and p53-mediated caspase-dependent apoptosis in nephrotoxicity of cisplatin. 2007; 12: 1901-1909.
- Al-Rasheed N.M., Al-Rasheed N.M., Bassiouni Y.A., Hasan I.H., Al-Amin M.A., Al-Ajmi H.N., Mahmoud A.M. Simvastatin ameliorates diabetic nephropathy by attenuating oxidative stress and apoptosis in a rat model of STZ-induced type 1 diabetes. Pharmacother. 2018; 105: 290-298.
- Meyer T.W., Bennett P.H., Nelson R.G. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. 1999; 42: 1341-1344.
- Steffes M.W., Schmidt D., McCrery R., Basgen J.M. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001; 59: 2104-2113.
- Ahmed O.M., Ali T.M., Abdel Gaid M.A., Elberry A. Effects of enalapril and paricalcitol treatment on diabetic nephropathy and renal expressions of TNF-α, P53, caspase-3 and Bcl-2 in STZ-induced diabetic rats. bioRxiv 2019;2019: 1-33.
- JäpeltB., Jakobsen J. Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013; 4: 1-20.
- Fischer H., Koenig U., Eckhart L., Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem. Biophys. Res. Commun. 2002; 293: 722–726.
- Lüthi A.U., Martin S.J. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 2007; 14: 641-650.
- Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: controlled demolition at the cellular level. Rev. Mol. Cell Biol. 2008; 9: 231-241.
- Kurokawa M., Kornbluth S. Caspases and kinases in a death grip. Cell. 2009; 138: 838-854.
- Sznarkowska A., Olszewski R., Zawacka-Pankau J. Pharmacological activation of tumor suppressor, wild-type p53 as a promising strategy to fight cancer. Postepy Hig. Med. Dosw. 2010;64: 396-407
- El-Beshbishy H.A., Bahashwan S.A., Aly H.A., Fakher H.A. Abrogation of cisplatin-induced nephrotoxicity in mice by alpha lipoic acid through ameliorating oxidative stress and enhancing gene expression of antioxidant enzymes. J. Pharmacol. 2011; 668: 278-284.
- Zhang S., Ong C.N., Shen H.M. Involvement of proapoptotic Bcl-2 family members in parthenolide-induced mitochondrial dysfunction and apoptosis. Cancer Lett. 2004; 211: 175-188.